Buvidal safety and efficacy
The clinical research behind Buvidal
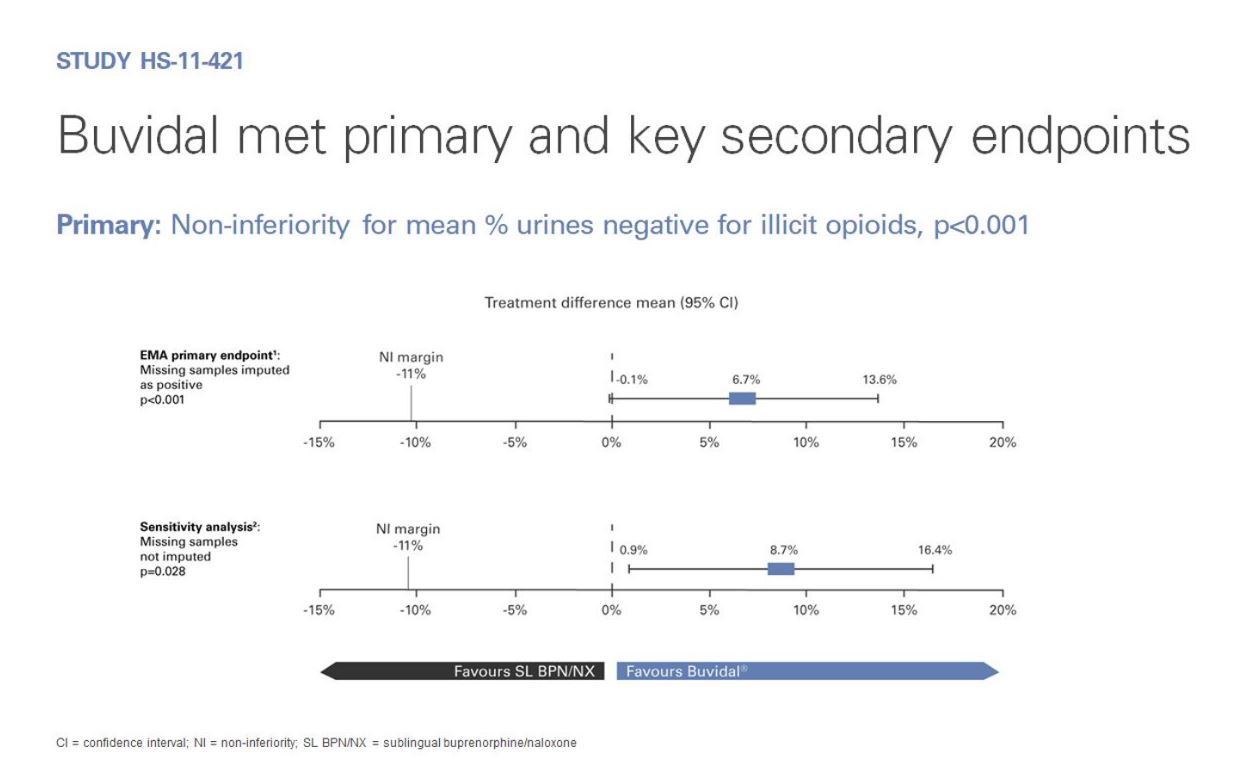
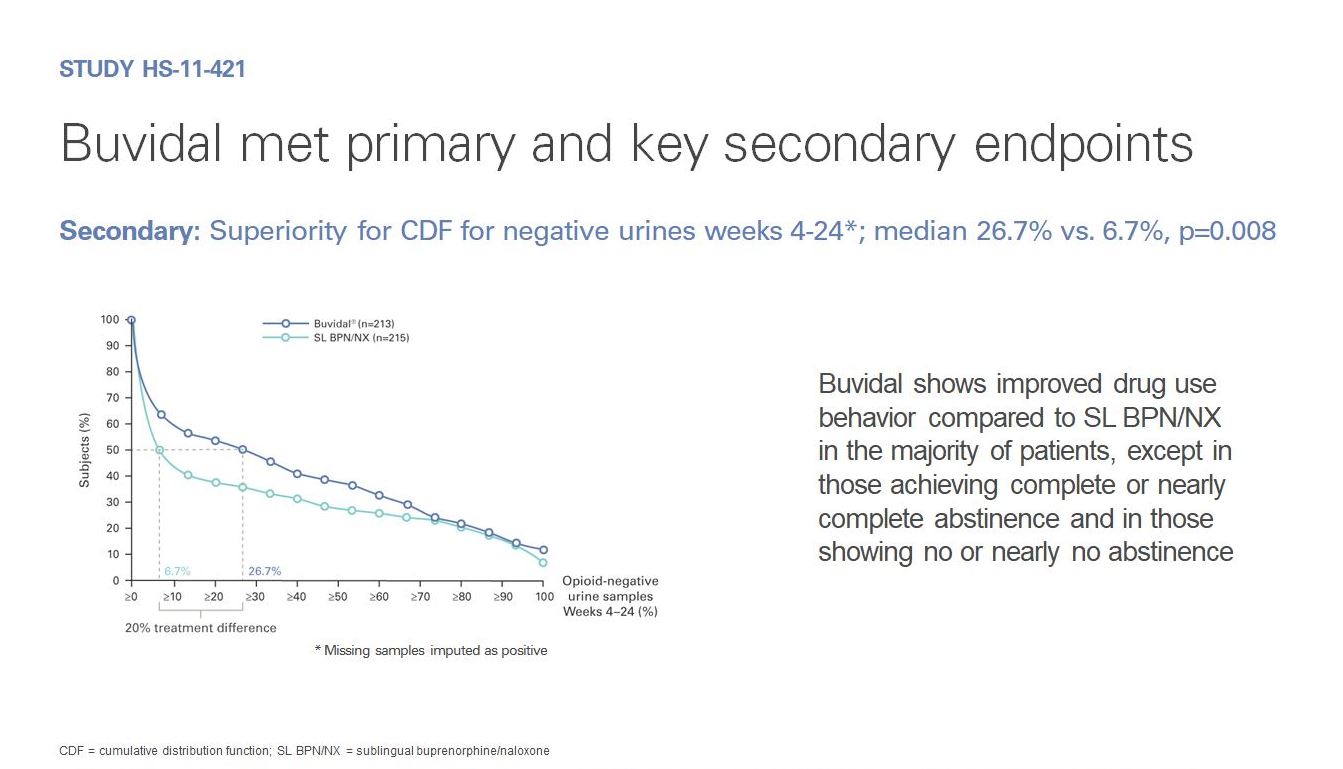
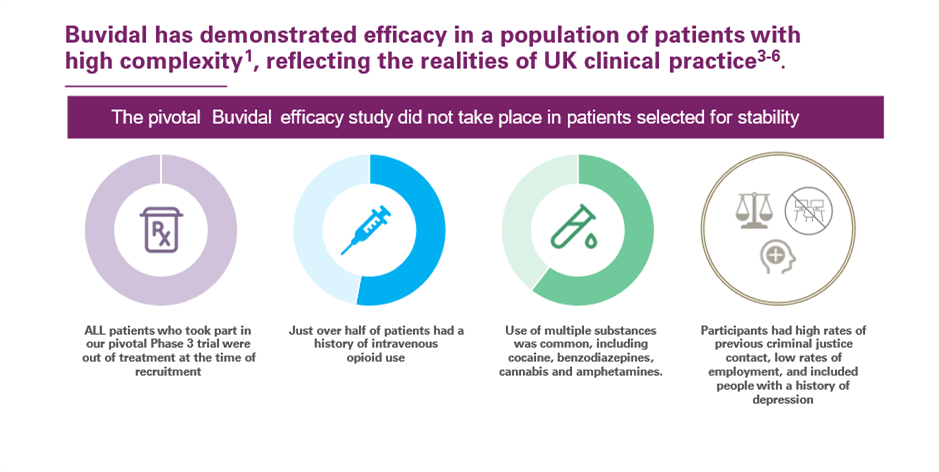
As part of its clinical development programme, the safety and efficacy of Buvidal in the treatment of opioid dependence have been investigated in two phase 3 studies.
These studies were carried out with patients with moderate to severe opioid dependence to reflect the likely treatment population.
Phase 3 double dummy, double blind efficacy study
The efficacy and safety profile of Buvidal in the treatment of opioid dependence were established in a pivotal phase 3, randomised, double-blind, double-dummy, active-controlled, flexible-dose study in patients with moderate to severe opioid dependence. In this study, 428 patients were randomised to one of two treatment groups.
Phase 3 open-label, long-term safety study
A long-term, open-label, phase 3 safety study with flexible dosing of weekly and monthly Buvidal for 48 weeks was conducted.