The only weekly and monthly long-acting
individualised treatment for opioid dependence¹
About Buvidal
This section is intended for healthcare professionals prescribing or administering Buvidal for treatment of patients with opioid dependence.
We strongly recommend that the information is read alongside the Summary of Product Characteristics (SmPC) and Patient Information Leaflet/package insert (PIL). These can be found on the Electronic Medicines Compendium (EMC) website medicines.org.uk.
Product Overview
Buvidal is available in weekly and monthly formulations.
These in turn are available in four doses as follows:
- Weekly Buvidal is available in 8,16, 24 and 32 mg doses
- Monthly Buvidal is available in 64, 96,128 and 160 mg doses
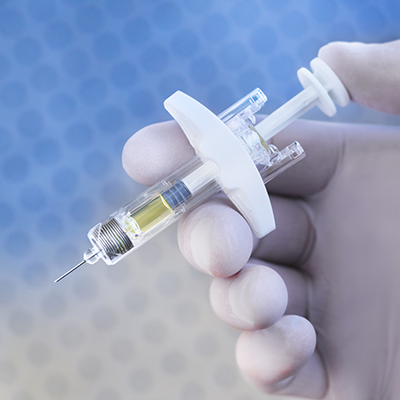
Both weekly and monthly Buvidal come in the form of a single use, pre-filled syringe for subcutaneous injection.
The injection must be administered by a qualified healthcare professional.
In the interests of healthcare professional and patient safety, the syringe features a safety device to prevent accidental needlestick injury post-injection.
Buvidal does not require cold storage and can be kept in a conventional controlled drugs cabinet. It has as shelf-life of three years.