Pre-filled syringe with needlestick protection
Administration
Administering Buvidal
Buvidal can only be administered by a qualified healthcare professional – it is not suitable for take home use or self-administration.
It is licensed for subcutaneous injection only – it must not be administered intravascularly (intravenously), intramuscularly or intradermally.
Intravascular administration, such as intravenous injection, would present a risk of serious harm as Buvidal forms a solid mass upon contact with body fluids which could potentially cause blood vessel injury, occlusion or thromboembolic events.
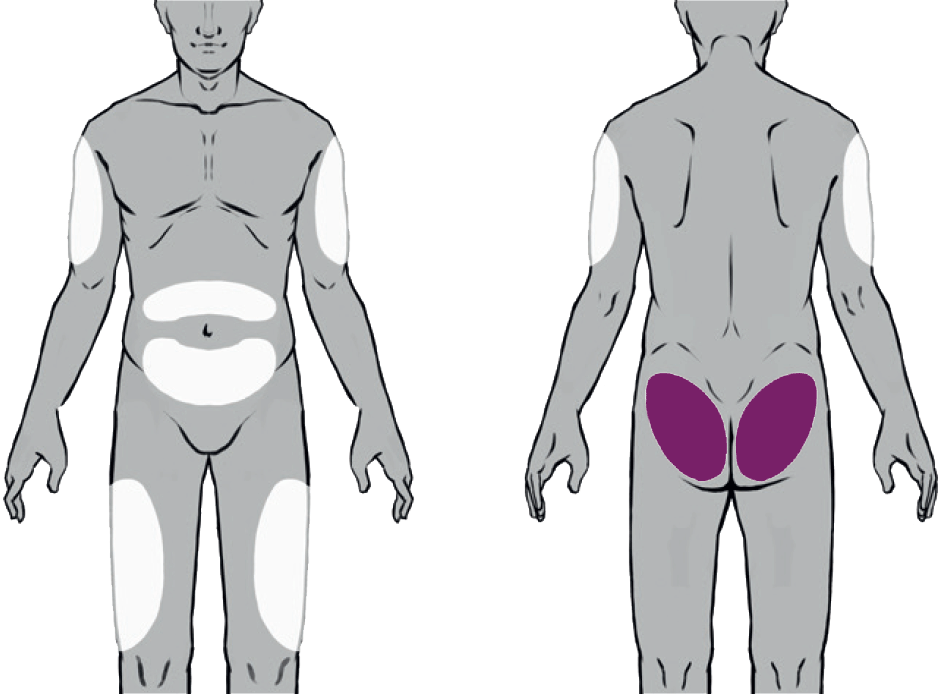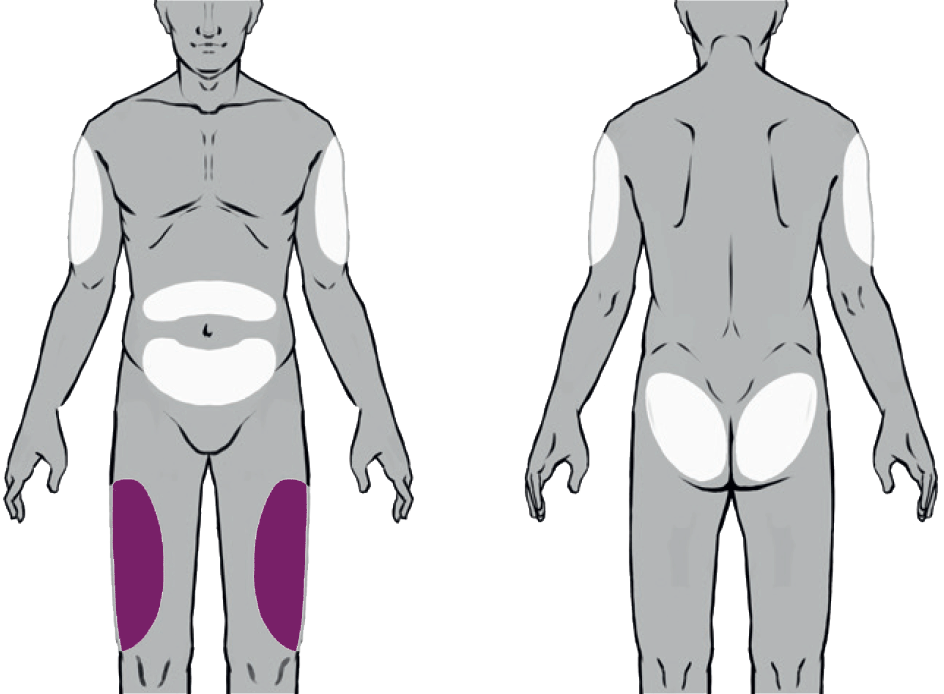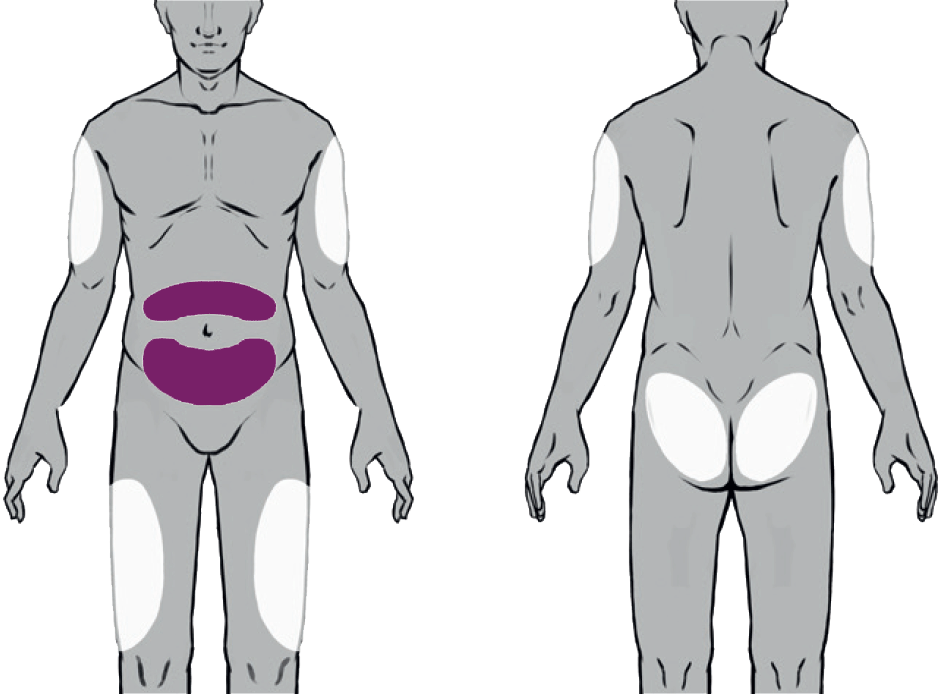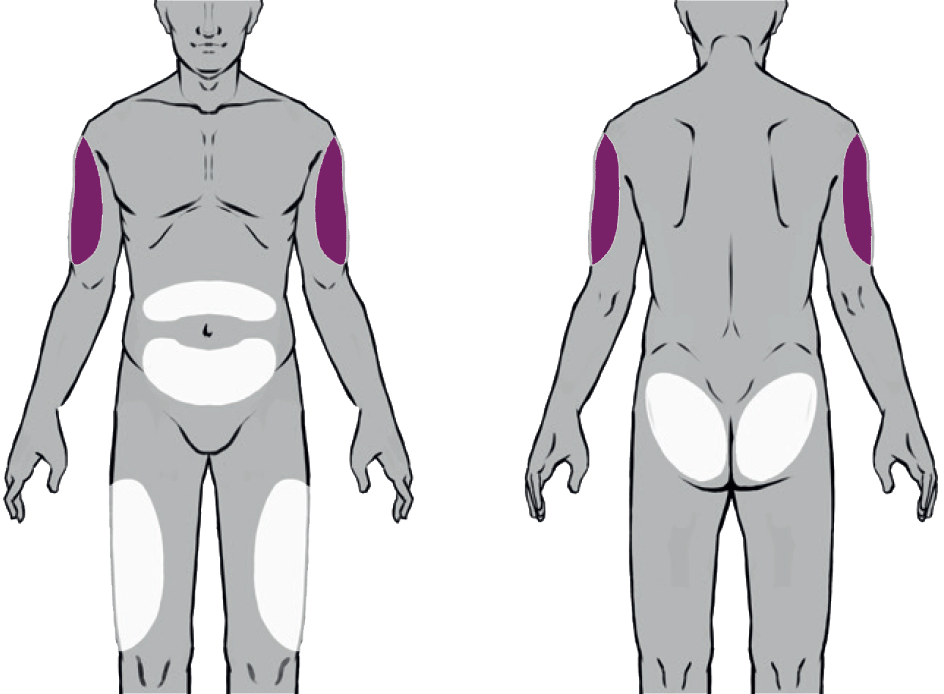
Suitable Injection Sites
Buvidal can be administered into one of eight different injection sites (thighs, buttocks, upper arms or abdomen) as shown in the diagram, provided there is sufficient subcutaneous tissue. Buvidal should be injected slowly and completely as a singe injection into the chosen site.
Injections on the waistline or within 5 cm of the navel should be avoided as tight clothing could irritate the injection site.
Injection sites should be rotated for both weekly and monthly injections. A minimum of 8 weeks should be left before re-injecting a previously used injection site with the weekly dose.
There is no clinical data supporting reinjection of the monthly dose into the same site. This is unlikely to be a safety concern. The decision to reinject at the same site should ultimately be guided by the HCP’s own clinical judgement.
There are 8 potential injection sites:
For Your Protection
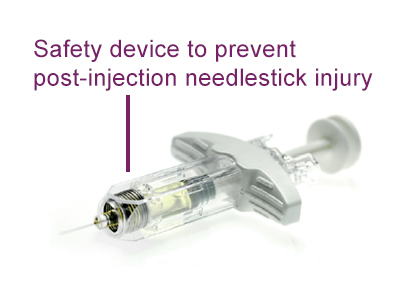
The Buvidal pre-filled syringe incorporates a needle-safe device to minimise the risk of exposure to blood-borne viruses that could arise from an accidental needlestick injury.
After administering Buvidal and withdrawing the needle from the patient, the HCP simply needs to take their thumb off the plunger and the syringe guard will automatically cover the exposed needle. The entire syringe can then be disposed of in a suitable sharps container.
How to inject Buvidal User Guide
Watch a short video that explains how to administer Buvidal
Precautions When Administering Buvidal
- Always check you are using the correct prescribed dose
- Do not use the safety syringe after the expiration date shown on the cardboard box or on the syringe label
- The needle shield of the syringe may contain rubber latex that may cause allergic reactions in latex sensitive individuals
- Inspect the safety syringe closely:
- The product must not be used if the safety syringe is broken or the packaging is damaged
- A small air bubble may be seen, which is normal
- The liquid should be clear. Do not use the safety syringe if the liquid contains visible particles or is cloudy
- Handle the safety syringe carefully to avoid a needlestick injury. The safety syringe includes a needle protection safety device that will activate at the end of the injection
- Do not uncap the safety syringe until you are ready to inject.
- Once uncapped, never try to recap the needle
- Dispose of the used safety syringe right away after use. Do not re-use the safety syringe
- Any unused medicinal product or waste material should be disposed of in accordance with local requirements

